
Developing local-acting immunotherapies


Developing local-acting immunotherapies
Ilya Pharma, is developing novel therapeutic modalities to catalyze the future of local-acting immunotherapies and fighting disease by local instructions to immune cells for various clinical applications including immune modulation and addressing the WHO critical MDR pathogens.
Ilya Pharmas approach is inspired by the early work of Professor Ilya Mechnicov who also received the Nobel Prize in 1908, describing immune cells have multiple functions in vivo, read more here.
Mia’s election recognises her outstanding contributions — both in her research in hashtag#physiology founding Ilya Pharma and in hashtag#driving hashtag#innovation at the interface of science, business and healthcare. Her voice will now join IVA’s distinguished network of thinkers and doers shaping the future of Swedish industry and innovation. IVA — the world’s oldest academy of engineering sciences, which unites around 1,300 leading engineers, researchers, entrepreneurs and business leaders to advance technology, industry and sustainable societal development, see more at iva.se
Lofton Tomenius H et al., (2025) Elimination of highly multidrug-resistant wound bacteria by the lactic acid bacterial drug candidate ILP100. Infectious Disease and Therapy
Öhnstedt E et al., (2024) Oral administration of CXCL12 expressing Limosilactobacillus reuteri improves colitis by local immunomodulatory actions in preclinical models. American Journal of Physiology – Gastrointestinal and Liver Physiology
Mishra A, E. (2024) Localized immunotherapy for colitis: breakthroughs with CXCL12-expressing Limosilactobacillusreuteri. American Journal of Physiology – Gastrointestinal and Liver Physiology
Liu, H et al., (2021) Distinct B Cell Subsets in Peyer’s patches Convey probiotic effects by Limosilactobacillus reuteri. Microbiome 9(198).
Öhnstedt, E et al., (2023) Engineered bacteria to accelerate wound healing: an adaptive, randomized, double-blind, placebo-controlled, first-in-human phase 1 trial. eClinicalMedicine a Lancet Discovery Science Journal.
Öhnstedt, E et al., (2022) Accelerated wound healing in minipigs by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Pharmaceutics 14(2):229.
Vågesjö, E et al., (2018). Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proceedings of the National Academy of Sciences, 115(8), 1895–1900.
Karimi, S et al., (2016). In Vivo and In Vitro Detection of Luminescent and Fluorescent Lactobacillus reuteri and Application of Red Fluorescent mCherry for Assessing Plasmid Persistence. PLOS ONE, 11(3), e0151969.
Phillipson, M., and Kubes, P., (2019) The Healing Power of Neutrophils. Trends in Immunology Jul;40(7):635-647.
Vågesjö, E et al., (2021). Perivascular Macrophages Regulate Blood Flow Following Tissue Damage. Circulation Research May;128(11):1694-1707.
Ilya Pharma's lead programs address (a) colitis especially cancer patients receiving ICI therapy and (b) life threatening difficult-to-treat skin wounds in patients with a rare genetic condition (SAVI) affecting the interferon signalling pathway STING and (c) on preserving lung function in patients with rare and genetic diseases, e.g. SAVI and cystic fibrosis.
Ilya’s technology leverages pharmaceutical development of well known, but biochemically unstable, human therapeutic proteins acting on tissue immune cells to be produced locally exactly where they are needed by live non-human, non-colonizing lactic acid bacteria acting as local mini-bioreactors.
The modality is new and allows the use of a wide range of immune active proteins that are not suited to be developed as traditional biologics or gene therapy and in a very cost-efficient way and is classified as GTMP and gene therapy by the EMA and FDA respectively.
The platforms competitive advantages include:
-Unlocks drugging of > 1700 proteins
-Favourable safety profile(s)
-Manufactured at large scale
-Low COGs/potential for competitive margin
-Logistics and storage at ambient temp
-Opportunity to address wide range of disease in skin and mucosa
ILP100 is the lead asset and is more specifically L. reuteri expressing human CXCL12 1a. The INN is emilimogene sigulactibac. ILP100 is developed in an Oral and Topical formulations.
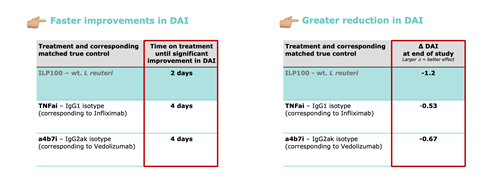
ILP100-Oral has completed the non-clinical development showing very favourable safety profile and in animal models of colitis demonstrated superior efficacy compared to marketed benchmarks anti-TNFa(Infliximab) and anti-a4b7 (Vedolizumab). A preIND meeting with the FDA was held and the FDA supports evaluation of ILP100-Oral as first line treatment in mild and moderate coltis in cancer patiens caused by the immune check point inhibitors (ICIs).
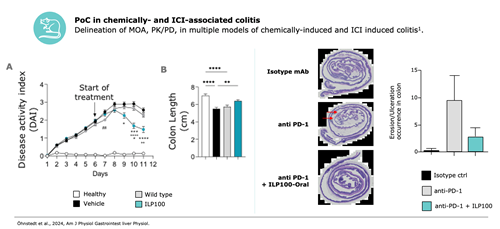
ILP100 outperforms benchmark controls for the marketed products Remicade and Entyvio
All, key non-clinical data from Öhnstedt et al., 2024 AJP.
ILP100-Topical has undergone extensive drug profiling with successful regulatory interactions and approvals for phase 1 and 2/3 trials in EU (EMA/CAT, Swedish MPA) and US (FDA/CEBR), a first in human double blinded RCT trial showing favourable safety profile and significantely 10 days faster skin healing. An IND is open in the US and a clinical trial in pediatric patients with the genetic disease SAVI have ben planned together with experts at interferonophaties at the NIH, FDA have granted an Rare Paediatric Disease Designation (RPDD) for SAVI. Regulatory compliance with approval processes been vetted with Indian CDSCO and Chinese NMPA by potential partners for other undisclosed indications.
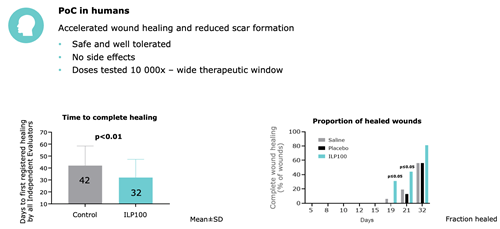
Key clinical data from double-blinded RCT from Öhnstedt et al 2023 EClinicalMedicine Lancet Science Discovery.
ILP101-Inhale is in non-clinical proof-of concept stage and is more specifically L. reuteri expressing human CXCL17, a mucosal chemokine with anti-inflammatory and bacteria killing effects. The program is currently advanc performed together with academic partners, and grant funded by EU Marie Sklodovska Curie program B-ACTIVE and the Swedish Cystic Fibrosis Foundation.
Ilya Pharma’s lead assets are complemented by a pipeline of non-disclosed assets addressing AMR/MDR for the yet untapped opportunity of eliminating pan-resistant bacterial infections with lactic acid bacteria.
Ilya Pharma conducts its own development, with GMP/GCP in house capabilities as well as in partnership with other renowened international thought leaders and organisation. The long-term relationships with European Innovation Council, National Institure for Health, Uppsala University, Swedsih Univerity of Agriculture and Katolishe University of Leuven are such examples.
The different regulatory pathways for accelerated approvals for innovative Cell- and Genetherapeis including the FDA RPDD and RMAT and EMA PRIME significantely de-risks and limits investments needed into the developments until approval with indicative values in the range of >100 mUSD.
Immune Checkpoint Inhibitor induced Colitis
Up to 58% of cancer patients receiving immune check point inhibitors (ICIs) develop gastrointestinal toxicities and cause of death is most often ruptures leading to sepsis, sepsis with multi drug resistant bacteria is >2.5x more common in cancer patients. With ILP100-Oral Ilya Pharma is seeking the clinical PoC for a new first-line-treatment in patients with ICI-induced colitis in a multicenter first-in-human phase 1/2a, the trial enrolling healthy subjects and cancer patients on ICIs suffering from ICI-induced colitis stage 1 and 2. The main hypothesis, and driver of value, is to allow the immune system to be aggressive fighting the cancer, as induced by the ICIs - but leaving the gut alone using ILP-100-oral as a local acting anti-inflammatory treatment. The current standard of care for ICI-induced colitis relies solely on systemic anti-inflammatory treatments being steroids and biologics and these come with a number of drawbacks such as their adverse event profiles and counteracting the anti-cancer effects of the ICIs. The ultimate goal is to facilitate a larger portion of patients on ICI treatment to be able to complete the lifesaving treatment.
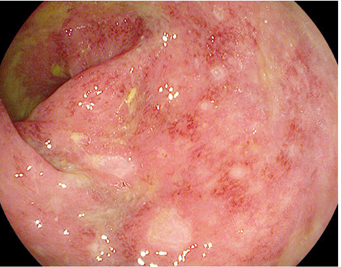
Colon with ICI-induced colitis where especially fibrosis is visible, Figure from Som et al., 2019 World J Clin Cases
Targeted Patient and Payer benefits:
1) ILP100-oral empowers ICI continuation in patients developing ICI-colitis and responding well to the cancer treatment
2) ILP100-oral eliminates the issue of systemic immunosuppression counteracting the ICI-treatment objectives
3) ILP100-oral avoids AEs typical for systemic immunosuppressants and reduces infection risk
4) ILP100-oral makes ICI-treatment available to a wider group, improving treatment equity
5) ILP100-oral enables increased cost efficiency for payers
SAVI (STING-Associated Vasculopathy with Onset in Infancy) - skin phenotype
In SAVI patients, gain-of-function mutations in the STING1 gene drive constitutive type I interferon signalling, resulting in chronic inflammation, vasculopathy and high morbidity. Approximate 1/3 or the patients have a severe skin phenotype with ulcerations, infections and repeated amputations. Conventional immunosuppressive therapies and JAK inhibitors incompletely address skin lesions, leaving a significant unmet need for interventions that promote local tissue restoration. Preclinical and early clinical studies of ILP100 demonstrate accelerated wound healing via sustained local CXCL12 delivery, increased TGF-β-expressing macrophages, improved local blood flow, and antimicrobial activity against multidrug-resistant wound pathogens. These properties suggest that ILP100 has the potential to promote wound closure, limit tissue loss, and reduce infection risk in SAVI-related skin ulcers, supporting its clinical evaluation in this patient population. FDA have granted an Rare Paediatric Disease Designation (RPDD) for SAVI and the ILP100-Topical and Ilya Pharma have planned a confirmatory clinical trial with the patient organisation, the KOLs and lead sites, NIH, uPenn, Great Ormond Street Hospital and more. "An Open Label Dose Escalation Exploratory Phase 2 Study to Evaluate Safety and Biological Effect of ILP100-Topical in Subjects with STING-Associated Vasculopathy with Onset in Infancy and Skin Ulcers on Wound Healing and Limb Saving".
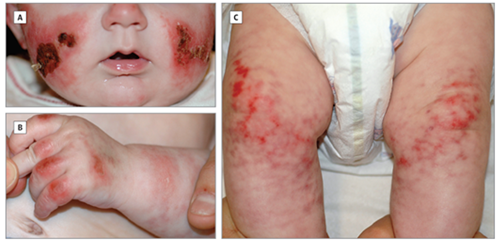
Typical skin wounds in paediatric patient with SAVI, Figure from Munoz et al., 2015 JAMA Dermatology
Targeted Patient and Payer benefits:
1) stabilization of target wound, 2) limiting tissue necrosis, 3) increasing probability of limb preservation (limb-saving) and 4) killing MDR bacterial pathogens
SAVI with lung phenotype and Cystic Fibrosis
In SAVI patients, gain-of-function mutations in the STING1 gene drive constitutive type I interferon signalling, resulting in chronic inflammation, vasculopathy and high morbidity. Approximate 1/3 or the patients have a severe lung phenotype. Respiratory symptoms in SAVI can be insidious and are not specific (chronic cough, exertional dyspnea, and hemoptysis) and pulmonary function tests shows mainly a restrictive syndrome with diffusion impairment, sometimes associated with hyperinflation, but also obstruction or a mixed lung function impairment and biopsies shows increased inflammation and fibrosis. Repeated infections are common and problems with chronic multi drug resistant bacteria and may lead to the need of a lung transplant.
Cystic Fibrosis is a well studies genetic rare disease and patients are have recently experienced benefits from CTFR "correct and enhance" combination therapy. There is stilla significant treatment gap in further preserving or improving the declining lung function and adress the often chronic lung infection with MDR pathogens.
The overall aim of this project is to evaluate a novel, local bacterial immunotherapy based on Limosilactobacillus reuteri transformed to produce and release the chemokine CXCL17 for the treatment of inflammation and multidrug-resistant (MDR) bacterial infections in models of STING-Associated Vasculopathy with onset in Infancy (SAVI) and cystic fibrosis (CF). CXCL17 belongs to a group of mucosal chemokines where exact functions in homeostatis and disease are being uncovered.
By delivering high local concentrations of CXCL17 directly to the respiratory mucosa, the therapy is designed to:
1) Reduce inflammatory neutrophil recruitment
2) Enhance neutrophil- and macrophage-mediated bacterial clearance,
3) Leverage the intrinsic antimicrobial effects of both CXCL17 and L. reuteri, and
4) Promote resolution of inflammation and tissue repair in the subepithelial lung tissue.
5) Preserve measurable, meaningful lung function following an infection
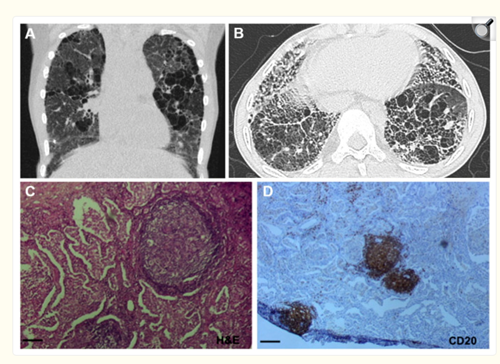
Representative Lung Imaging and Pathology in SAVI showing fibrosis and inflammation from David and Frémond 2022 Cells.
The current major shareholders include institutional investor EIC Fund, 5 bn EUR fund, investing exclusively in innovative and disruptive technologies aiming to make significant impact, and four family offices represented by a single point of contact.
Marie Skłodowska Curie Activities funded by the European Union. The B-ACTIVE program received funding from the European research and innovation program MARIE SKŁODOWSKA-CURIE ACTIONS Doctoral Networks – Horizon 2022 under grant agreement No. 101120187, 2024-2028
Together with Uppsala University, Swedish Cystic Fibrosis Foundation, 2026
H2020 SME phase 2 grant WHILYAs for first in human RCT phase 1 clinical trial of ILP100-Topical under Grant Agreement No 804438, EUR 3 million
EIC Accelerator grant WHILYAs-2 for clinical phase 2a study in DFU of ILP100-Topical under Grant Agreement No 960913, EUR 1.4 million
STUNS Bio-X funding for IND enabling- and formulation studies for ILP100-Oral, EUR 640 000.
EIT Health Start up grant and Grant for Options supporting key CMC activities, EUR 500 000.
National Grants from Vinnova SweLife Step 2, Innovative Startups and Tillväxtverket.
National Almi International Growth, Grant ID 20357664 from European Regional Development Fund
EU Grants

















Ilya Pharma AB, Uppsala Business Park, 752 37 Uppsala, Sweden
Ilya Pharma Inc, Delaware C corp registered for business in Houston